This month we continue our #epicfail series on failure mechanisms looking at the impact of operating below the dew point on materials exposed to products of combustion.
Sulphuric acid dew point corrosion or cold-end corrosion occurs due to the condensation of sulphuric acid and is commonly observed in components which are exposed to products of combustion i.e. a boiler or an engine. Sulphur is present in heavy oil and during combustion, sulphur oxides are generated, a small portion of which becomes SO3. As the exhaust gas temperature reaches the dew point or lower, vapour-phase sulphuric acid forms (1). If the sulphuric acid vapour contacts the lower-temperature metal surface it may condense as liquid sulphuric acid and corrode the metal according to the following reaction (2):
H2SO4 + Fe → FeSO4 + H2
The temperature at which sulphuric acid first condenses varies between 116 and 166°C or higher, depending on the sulphur trioxide and water vapour concentrations in the exhaust gas (2). The critical factors governing sulphuric acid dew point corrosion include the presence of sulphur trioxide, the presence of moisture, and the presence of metal whose surface temperature is below the sulphuric acid dew point. The dew point increases with sulphur trioxide content and moisture content (2).
Dilute sulphuric acid (5 to 40%) is more corrosive than higher concentrations and as the H2O dew point is approached, acid concentrations in this range are likely (3). Table 1 shows the content levels of corrosive components in the flue gas of plants burning different fuels, principal acid condensations and the ranges of their dew points (4).
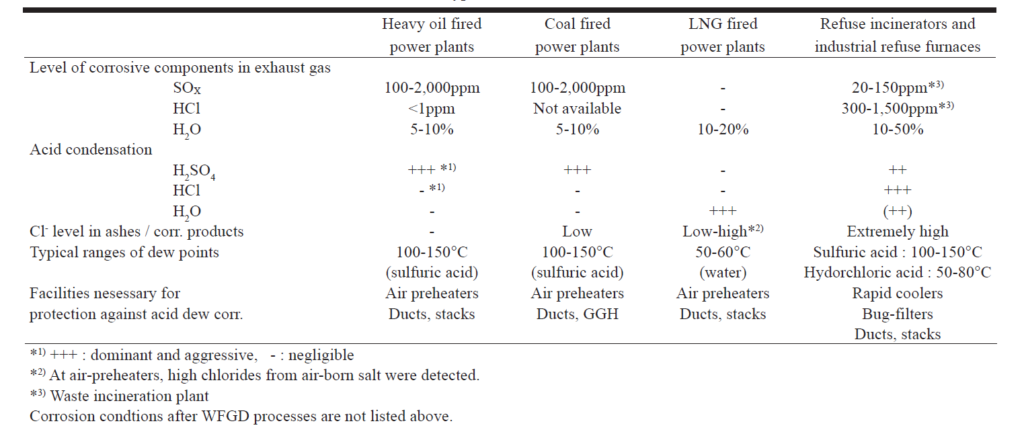
Sulphuric acid dew point corrosion typically produces a smooth metal surface, with distinct channels or grooves with a corrosion product consisting of iron sulphates. The consequences of such a mechanism can be disastrous due to its localised nature. As the wall thickness reduces so that the effective cross section can no longer withstand the applied stress, rupture can occur.


Maintaining temperatures above the dew-point of sulphuric acid will prevent corrosion of this type. Reducing the sulphur content of the fuel will help to minimise the occurrence of sulphuric acid dew point corrosion. Furthermore, in boilers, a powerful and efficient soot blowing system can reduce the likelihood of this mechanism.
- Nippon Steel & Sumitomo Metal. Sulphuric acid and hydrochloric acid dew-point corrosion resistant steel. S-TENTM
- The NALCO Guide to Boiler Failure Analysis. Robert D. Port and Harvey M. Herro. Cold end corrosion during service. Chapter 12. Page 143
- Materials Selection for Corrosion Control-Dew Point Corrosion. Materials Selection for Corrosion Control. S.L. Chawla and R.K. Gupta. ASM Handbook. 1993. Page 60
- New S-TENTM1: An Innovative Acid-Resistant Low Alloy Steel. Nippon Steel Technical Report No. 90 July 2004
Would you like to know more about our Failure Analysis services? Contact us today to find out more!